Daniotti et al, Traffic 2017
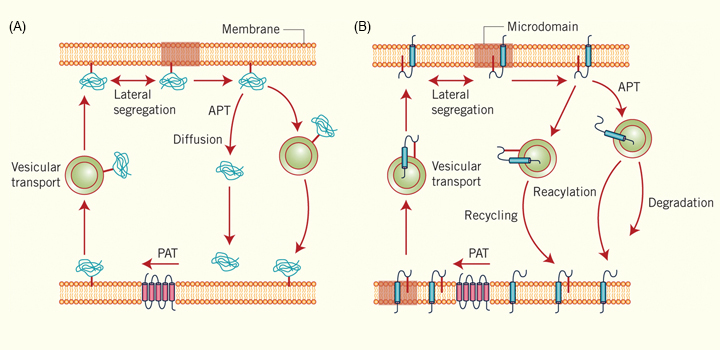
Protein S-acylation, also known as palmitoylation, consists of the addition of a lipid molecule to one or more cysteine residues through a thioester bond. This modification, which is widespread in eukaryotes, is thought to affect over 12% of the human proteome. S-acylation allows the reversible association of peripheral proteins with membranes or, in the case of integral membrane proteins, modulates their behavior within the plane of the membrane. This review focuses on the consequences of protein S-acylation on intracellular trafficking and membrane association. We summarize relevant information that illustrates how lipid modification of proteins plays an important role in dictating precise intracellular movements within cells by regulating membrane-cytosol exchange, through membrane microdomain segregation, or by modifying the flux of the proteins by means of vesicular or diffusional transport systems. Finally, we highlight some of the key open questions and major challenges in the field.
Authors: Daniotti JL, Pedro MP1,2, Valdez Taubas J
Article: Traffic. 2017 Nov;18(11):699-710. doi: 10.1111/tra.12510. Epub 2017 Sep 24.