Ruggiero FM et al. 2017, Biochim Biophys Acta.
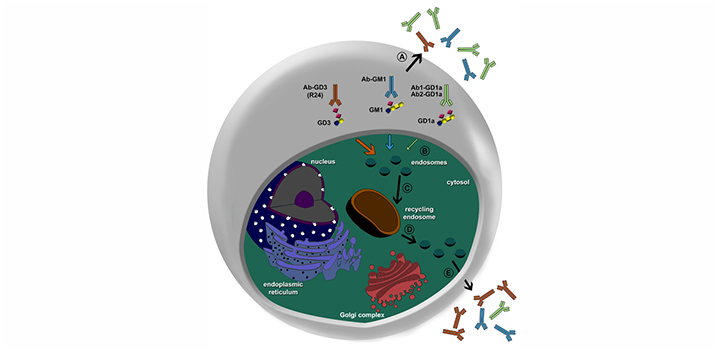
Antibodies against GM1 and GD1a gangliosides are associated with selective dysfunction of motor axons in peripheral neuropathies, and differential endocytic processing of antibodies to gangliosides represent a critical modulator of site-specific injury in Guillain-Barré syndrome. In addition, antibodies to glycolipids have emerged as an attractive tool for therapeutic interventions in cancer. In this work, we have investigated the binding, endocytosis and intracellular fate of high-affinity antibodies to gangliosides GD1a and GM1 both in epithelial and neuronal-like cells. Live cell imaging and fluorometric analysis showed that, after specific plasma membrane binding, a fraction of antibody to GD1a was slightly but rapidly internalized by a dynamin 2-independent pathway and then accumulated in the endocytic recycling compartment. We also show that internalization of antibody to GD1a is regulated by ADP-ribosylation factor 6. Surprisingly, experiment of cellular antibody uptake performed at 16 °C, widely used to accumulate the endocytic cargo in sorting endosomes, showed that the antibody to GD1a remained mostly localized at the plasma membrane, supporting the presence of selective mechanisms for cell internalization of antibody-ganglioside complex. In contrast, antibody to GM1 was endocyted in epithelial cells but remained at the plasma membrane of neuronal-like cells. Together, these results provide additional evidences about the molecular mechanisms that operate in the uptake and intracellular trafficking dynamics of antibodies to glycolipids and have significant translational implications for the understanding of clinical characteristics of anti-ganglioside antibody-mediated neuropathies and for the development of novel therapeutics targeting.
Authors: Ruggiero FM, Vilcaes AA, Yuki N, Daniotti JL.
Article: Ruggiero FM et al, Biochim. Biophys. Acta. Jan;1859(1):80-93. doi: 10.1016/j.bbamem.2016.10.020