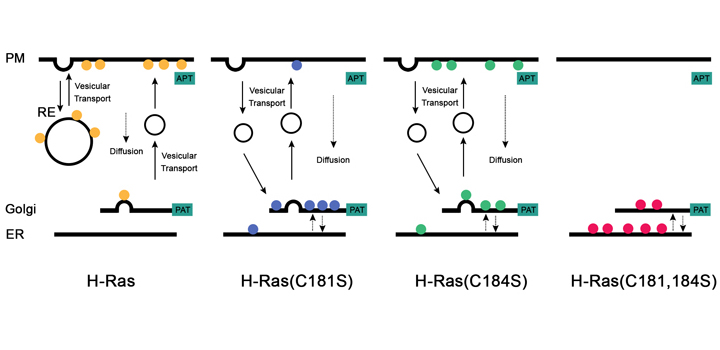
Pedro MP et al, Mol. Biol. Cell, 2017
S-acylation/deacylation cycles and vesicular transport are critical for an adequate subcellular distribution of S-acylated Ras proteins. H-Ras is dually acylated on cysteines 181 and 184, but it is unknown how these residues individually contribute to H-Ras trafficking. In this study, we characterized the acylation and deacylation rates and membrane trafficking of mono-acylated H-Ras mutants to analyze their contributions to H-Ras plasma membrane and endomembrane distribution. We demonstrated that dually acylated H-Ras interacts with acyl-protein thioesterases (APT) 1 and 2 at the plasma membrane. Moreover, single acylation mutants of H-Ras differed not only in their subcellular distribution, where both proteins localized to different extents at both the Golgi complex and plasma membrane, but also in their deacylation rates, which we showed to be due to different sensitivities to APT1 and APT2. Fluorescence photobleaching and photoactivation experiments also revealed that 1) although S-acylated, single acylation mutants are incorporated with different efficiencies into Golgi complex to plasma membrane vesicular carriers and 2) the different deacylation rates of single acylated H-Ras influence differentially its overall exchange between different compartments by non-vesicular transport. Taken together, our results show that individual S-acylation sites provide singular information about H-Ras subcellular distribution that is required for GTPase signaling.
Authors: Pedro MP, Vilcaes AA, Gomez GA, Daniotti JL.