Cejas et al. JBC 2018
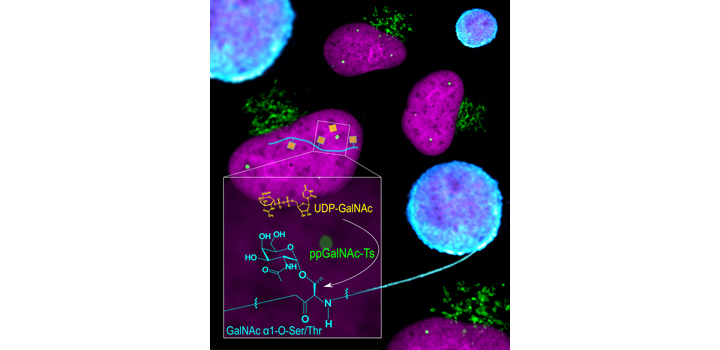
Biological functions of nuclear proteins are regulated by post-translational modifications (PTMs) that modulate gene expression and cellular physiology. However, the role of O-linked glycosylation (O-GalNAc) as a PTM of nuclear proteins in the human cell has not been previously reported. Here, we examined in detail the initiation of O-GalNAc glycan biosynthesis, representing a novel PTM of nuclear proteins, in the nucleus of human cells, with an emphasis on HeLa cells. Using soluble nuclear fractions from purified nuclei, enzymatic assays, fluorescence microscopy, affinity chromatography, mass spectrometry and FRET analyses, we identified all factors required for biosynthesis of O-GalNAc glycans in nuclei: the donor substrate (UDP-GalNAc), nuclear polypeptide GalNAc-transferase activity, and a GalNAc transferase (polypeptide GalNAc-T3). Moreover, we identified O-GalNAc glycosylated proteins in the nucleus and present solid evidence for O-GalNAc glycan synthesis in this organelle. The demonstration of O-GalNAc glycosylation of nuclear proteins in mammalian cells reported here has important implications for cell and chemical biology.
Authors: RB eyebrows, Lorenz V, YC Garay, Irazoqui FJ