CIQUIBIC-CONICET-UNC
Contacto : +54 351 5353855
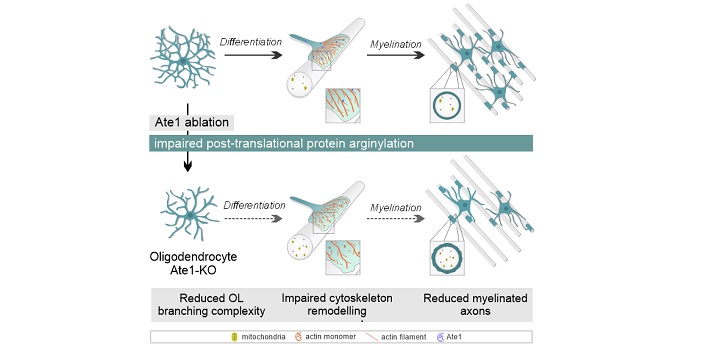
Addition of arginine (Arg) from tRNA can cause major alterations of structure and function of protein substrates. This post-translational modification, termed protein arginylation, is mediated by the enzyme arginyl-tRNA-protein transferase 1 (Ate1). Arginylation plays essential roles in a variety of cellular processes, including cell migration, apoptosis, and cytoskeletal organization. Ate1 is associated with neuronal functions such as neurogenesis and neurite growth. However, the role of Ate1 in glial development, including oligodendrocyte (OL) differentiation and myelination processes in the central nervous system, is poorly understood. The present study revealed a peak in Ate1 protein expression during myelination process in primary cultured OLs. Post-transcriptional downregulation of Ate1 reduced the number of OL processes, and branching complexity, in vitro. We conditionally ablated Ate1 from OLs in mice using 2′,3′-cyclic nucleotide 3′-phosphodiesterase-Cre promoter (“Ate1-KO” mice), to assess the role of Ate1 in OL function and axonal myelination in vivo. Immunostaining for OL differentiation markers revealed a notable reduction of mature OLs in corpus callosum of 14-day-old Ate1-KO, but no changes in spinal cord, in comparison with wild-type controls. Local proliferation of OL precursor cells was elevated in corpus callosum of 21-day-old Ate1-KO, but was unchanged in spinal cord. Five-month-old Ate1-KO displayed reductions of mature OL number and myelin thickness, with alterations of motor behaviors. Our findings, taken together, demonstrate that Ate1 helps maintain proper OL differentiation and myelination in corpus callosum in vivo, and that protein arginylation plays an essential role in developmental myelination.
Autores:
Artículo:
Palandri A., Bonnet L.V., Farias M.G., Hallak M.E:, Galiano M.R.. Glia. October 2021. https://doi.org/10.1002/glia.24107
Rafael G. Oliveira, Mariana Paolorossi, Leide Passos Cavalcanti, Antonio Malfatti-Gasperini, Guillermo G. Montich
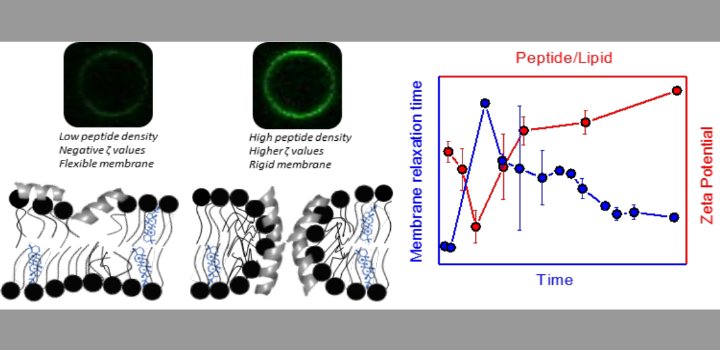
) for DP-containing LUVs showed a complex behavior at increasing peptide concentration. The effect of the peptide on membrane elasticity, investigated by nanotube retraction experiments, showed that peptide addition softened all membrane compositions, but membranes with DP got stiffer at long times. Considering this, and the ζ results, we propose that peptides accumulate at the interface adopting different arrangements, leading to a non-monotonic behavior. Possible correlations with cell membranes were inquired testing the antimicrobial activity of Polybia-MP1 against hopanoid-lacking bacteria pre-incubated with DP or CHO. The fraction of surviving cells was lower in cultures incubated with DP compared to those incubated with CHO. We propose that the higher activity of Polybia-MP1 against some bacteria compared to mammalian cells is not only related to membrane electrostatics, but also the composition of neutral lipids, particularly the hopanoids, could be important.
Keywords: Targeting of AMPs, Bacterial resistance, Membrane dynamic elasticity, Optical tweezers, Lytic activity, Zeta potential
Autores:
Artículo:
Alvares D.S, Monti M.R., Ruggiero Neto J., Wilke N. BBA Advances Volume 1, 2021, 100002. https://doi.org/10.1016/j.bbadva.2021.100002
There are several examples of single mutations that lead to a well-defined disease through a well-known mechanism. In other cases, a collection of mutations of the same protein produces a pathology with different degrees of severity. The accompanying work by Uusitalo et al. studies several mutants of the fatty acid binding protein P2 of the peripheral nervous system myelin. They conserve the native tertiary structure but a remarkable difference in the capacity to interact with lipids. This could be a clue to unravel the complex way in which these mutations affect myelin structure and function in a variant of Charcot-Marie-Tooth disease. Comment on: https://doi.org/10.1111/febs.16079.
Keywords: FABP; P2; emergence; myelin disorders; protein-lipid interaction.
Autores:
Artículo:
Pusterla J, Montich GG, Oliveira RG. Unraveling the etiology of myelin disorders: the P2 case in Charcot-Marie-Tooth disease. FEBS J. 2021 Jul 29. doi: 10.1111/febs.16131. Epub ahead of print. PMID: 34327848.