Gallea JI et al. 2016, Biochim Biophys Acta
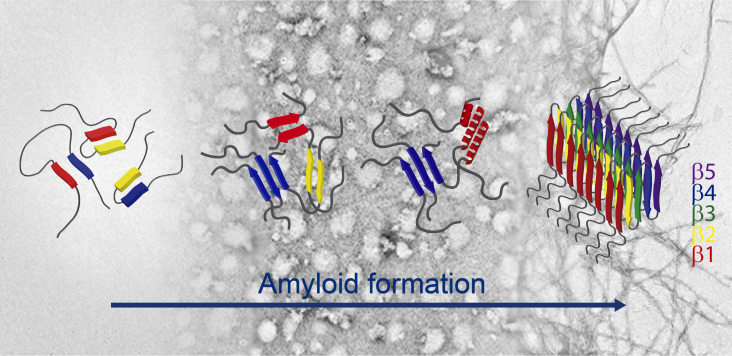
The misfolding and aggregation of the presynaptic protein α-synuclein (AS) into amyloid fibrils is pathognomonic of Parkinson’s disease, though the mechanism by which this structural conversion occurs is largely unknown. Soluble oligomeric species that accumulate as intermediates in the process of fibril formation are thought to be highly cytotoxic. Recent studies indicate that oligomer-to-fibril AS transition plays a key role in cell toxicity and progression of neurodegeneration. We previously demonstrated that a subgroup of oligomeric AS species are ordered assemblies possessing a well-defined pattern of intermolecular contacts which are arranged into a distinctive antiparallel β-sheet structure, as opposed to the parallel fibrillar fold. Recently, it was demonstrated that the physiological form of AS is N-terminally acetylated (Ac-AS). Here, we first showed that well-characterized conformational ensembles of Ac-AS, namely monomers, oligomers and fibrils, recapitulate many biophysical features of the nonacetylated protein, such as hydrodynamic, tinctorial, structural and membrane-leakage properties. Then, we relied on ATR-FTIR spectroscopy to explore the structural reorganization during Ac-AS fibrillogenesis. We found that antiparallel β-sheet transient intermediates are built-up at early stages of aggregation, which then evolve to parallel β-sheet fibrils through helix-rich/disordered species. The results are discussed in terms of regions of the protein that might participate in this structural rearrangement. Our work provides new insights into the complex conformational reorganization occurring during Ac-AS amyloid formation.
Autores: Gallea JI, Sarroukh R, Yunes-Quartino P, Ruysschaert JM, Raussens V, Celej MS
Artículo: Gallea JI et al, Biochim Biophys Acta. 2016 May;1864(5):501-10. doi: 10.1016/j.bbapap.2016.01.011