Dentesano YM et al. FEBS J. 2018
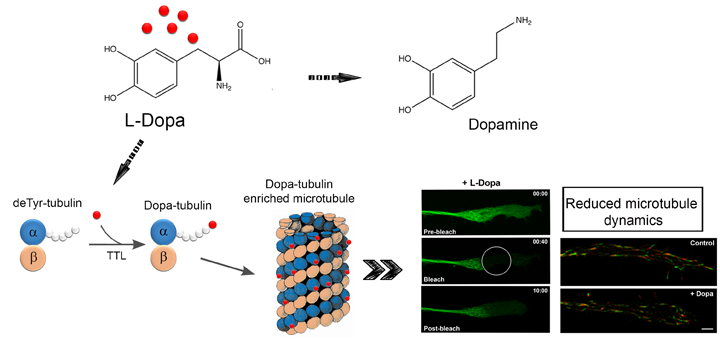
The C-terminal tyrosine (Tyr) of the α-tubulin chain is subjected to post-translational removal and readdition in a process termed the “detyrosination/tyrosination cycle”. We showed in previous studies using soluble rat brain extracts that l-3,4-dihydroxyphenylalanine (l-Dopa) is incorporated into the same site as Tyr. We now demonstrate that l-Dopa incorporation into tubulin also occurs in living cells. We detected such incorporation by determining the “tyrosination state” of tubulin before and after incubation of cells in the presence of l-Dopa. The presence of a tubulin isospecies following l-Dopa incubation that was not recognized by antibodies specific to Tyr- and deTyr-tubulin was presumed to reflect formation of Dopa-tubulin. l-Dopa was identified by HPLC as the C-terminal compound bound to α-tubulin. l-Dopa incorporation into tubulin was observed in Neuro 2A cells and several other cell lines, and was not due to de novo protein biosynthesis. Dopa-tubulin had microtubule-forming capability similar to that of Tyr- and deTyr-tubulin. l-Dopa incorporation into tubulin did not notably alter cell viability, morphology, or proliferation rate. CAD cells (a neuron-like cell line derived from mouse brain) are easily cultured under differentiating and nondifferentiating conditions, and can be treated with l-Dopa. Treatment of CAD cells with l-Dopa and consequent increase in l-Dopa-tubulin resulted in reduction of microtubule dynamics in neurite-like processes.
[/tp]
Autores: Dentesano YM, Ditamo Y, Hansen C, Arce CA, Bisig CG
Artículo: Dentesano YM et al, FEBS J. 2018 Mar;285(6):1064-1078. doi: 10.1111/febs.14386