Galassi VV et al, J. Phys. Chem. B, 2017
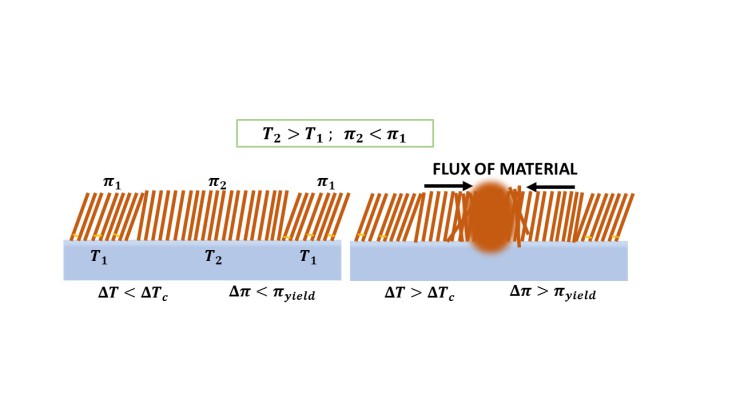
Langmuir monolayers of certain surfactants show a negative derivative of the surface pressure with respect to temperature. In these monolayers, a local temperature gradient leads to local yielding of the solid phase to a kinetically flowing liquid, so that the material flows toward the hotter regions that act as sinks. The accumulation of material leads to the formation of nonequilibrium multilamellar bubbles of different sizes. Here we investigate the molecular factors leading to such a peculiar behavior. First, we identify the required structural molecular moieties, and second we vary the composition of the subphase in order to analyze its influence. We conclude that esters appear to be unique in two key aspects: they form monolayers whose compression isotherms shift to lower areas as the temperature increases, and thus collapse into a hot spot; and they bind weakly to the aqueous subphase, i.e., water does not attach to the monolayer at the molecular level, but only supports it. Molecular simulations for a selected system confirm and help explain the observed behavior: surfactant molecules form a weak hydrogen bonding network, which is disrupted upon heating, and also the molecular tilting changes with temperature, leading to changes in the film density.
Autores: Galassi VV, Del Popolo MG, Fischer TM, Wilke N