Yunes Quartino et al. BBRC. 2018
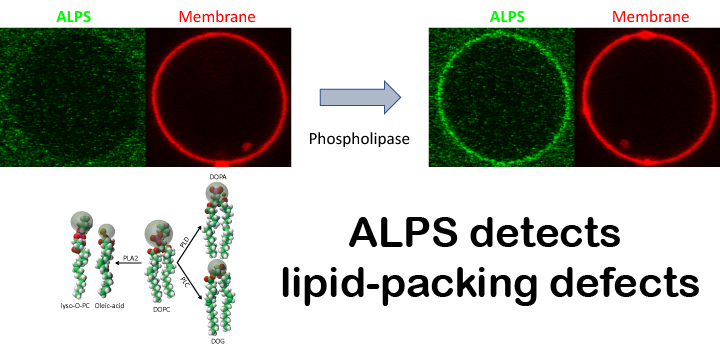
The amphipathic lipid packing sensor (ALPS) motif of ArfGAP1 brings this GTPase activating protein to membranes of high curvature. Phospholipases are phospholipid-hydrolyzing enzymes that generate different lipid products that alter the lateral organization of membranes. Here, we evaluate by fluorescence microscopy how in-situ changes of membrane lipid composition driven by the activity of different phospholipases promotes the binding of ALPS. We show that the activity of phospholipase A2, phospholipase C and phospholipase D drastically enhances the binding of ALPS to the weakly-curved membrane of giant liposomes. Our results suggest that the enzymatic activity of phospholipases can modulate the ArfGAP1-mediated intracellular traffic and that amphiphilic peptides such as the ALPS motif can be used to study lipolytic activities at lipid membranes.
Autores: Quartino PY, Fidelio GD, Manneville JB, Goud B, Ambroggio EE.