González MC et al. 2016, FEBS Lett.
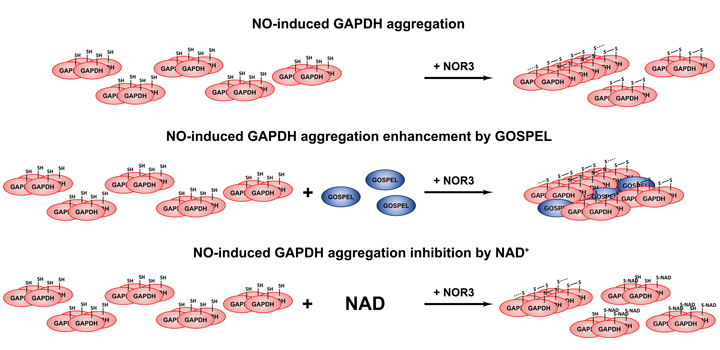
Glyceraldehyde-3-phosphate dehydrogenase’s (GAPDH’s) competitor of Siah Protein Enhances Life (GOSPEL) is the protein that competes with Siah1 for binding to GAPDH under NO-induced stress conditions preventing Siah1-bound GAPDH nuclear translocation and subsequent apoptosis. Under these conditions, GAPDH may also form amyloid-like aggregates proposed to be involved in cell death. Here, we report the in vitro enhancement by GOSPEL of NO-induced GAPDH aggregation resulting in the formation GOSPEL-GAPDH co-aggregates with some amyloid-like properties. Our findings suggest a new function for GOSPEL, contrasting with its helpful role against the apoptotic nuclear translocation of GAPDH. NAD+ inhibited both GAPDH aggregation and co-aggregation with GOSPEL, a hitherto undescribed effect of the coenzyme against the consequences of oxidative stress.
Autores: González MC, Romero JM, Ingaramo MC, Muñoz Sosa CJ, Curtino JA, Carrizo ME
Artículo: González MC et al, FEBS Lett. 2016 Jul;590(14):2210-20. doi: 10.1002/1873-3468.12242.