Socas LBP. BBA-Biomem. 2020
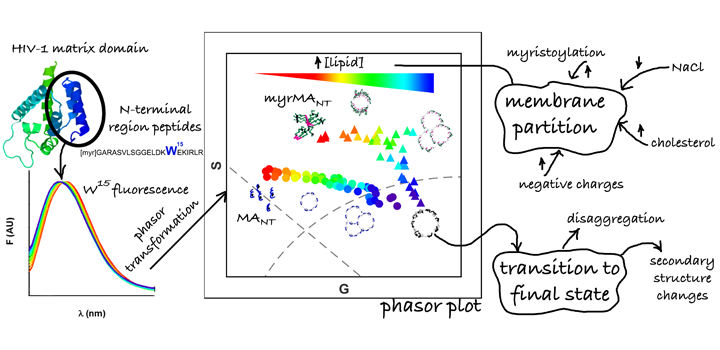
The group-specific antigen (GAG) polyprotein of HIV-1 is the main coordinator of the virus assembly process at the plasma membrane (PM) and is directed by its N-terminal matrix domain (MA). MA is myristoylated and possess a highly basic region (HBR) responsible for the interaction with the negative lipids of the PM, especially with PIP2. In addition, MA binds RNA molecules proposed as a regulatory step of the assembly process. Here we study the interaction of a synthetic peptide (N-terminal 21 amino acids of MA) and liposomes of different compositions using a variety of biophysical techniques. Particularly, we use the fluorescence properties of the single tryptophan of the peptide to analyze its partition to membranes, where we harness for first time the analytical ability of spectral phasors method to study this interaction. We found that electrostatic interactions play an important role for peptide partition to membranes and myristoylation reduces the free energy of the process. Interestingly, we observe that while the presence of PIP2 does not cause measurable changes on the peptide-membrane interaction, the interaction is favored by cholesterol. Additionally, we found that the partition process goes through a transition state involving peptide disaggregation and changes in the peptide secondary structure. On the other hand, we found that the presence of oligonucleotides competes with the interaction with lipids by increasing peptide solubility. In summary, we think that our results, in context of the current knowledge of the role of HIV-1 MA, contribute to a better molecular understanding of the membrane association process.
Authors: Socas LBP, Ambroggio EE
Article: The influence of myristoylation, liposome surface charge and nucleic acid interaction in the partition properties of HIV-1 Gag-N-terminal peptides to membranes. Socas LBP, Ambroggio EE. Biochim Biophys Acta Biomembr. 2020 Jul 22:183421. doi: 10.1016/j.bbamem.2020.183421 RTP Live