V Lorenz et al. 2016, J. Biol. Chem.
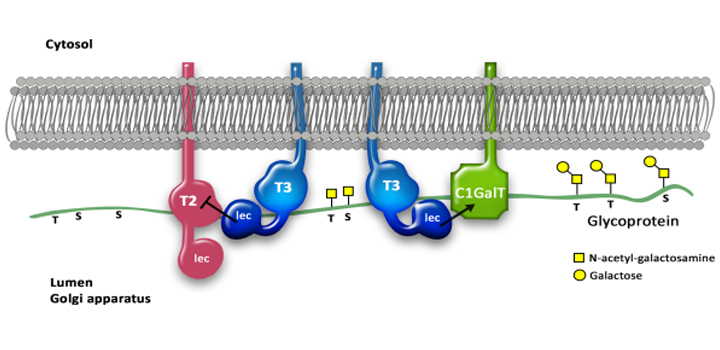
Glycan biosynthesis occurs mainly in Golgi. Molecular organization and functional regulation of this process are not well understood. We evaluated the extrinsic effect of lectin domains (β-trefoil fold) of polypeptide GalNAc-transferases (ppGalNAc-Ts) on catalytic activity of glycosyltransferases during O-GalNAc glycan biosynthesis. The presence of lectin domain T3lec or T4lec during ppGalNAc-T2 and ppGalNAc-T3 catalytic reaction had a clear inhibitory effect on GalNAc-T activity. Interaction of T3lec or T4lec with ppGalNAc-T2 catalytic domain was not mediated by carbohydrate. T3lec, but not T2lec and T4lec, had a clear activating effect on Drosophila melanogaster core 1 galactosyltransferase enzyme activity and a predominant inhibitory effect on in vivo human core 1 glycanbiosynthesis. The regulatory role of the β-trefoil fold of ppGalNAc-Ts in enzymatic activity of glycosyltransferases involved in the O-glycan biosynthesis pathway, described here for the first time, helps clarify the mechanism of biosynthesis of complex biopolymers (such as glycans) that is not template-driven.
Authors: Lorenz V, And Ditamo, RB eyebrows, Carrizo ME, Bennett EP, Clausen H, Nores GA, Irazoqui FJ.
Article: V Lorenz et al, J. Biol. Chem. 2016 Dec 2;291(49):25339-25350