Goitea VE et al. 2015, J. Biol. Chem.
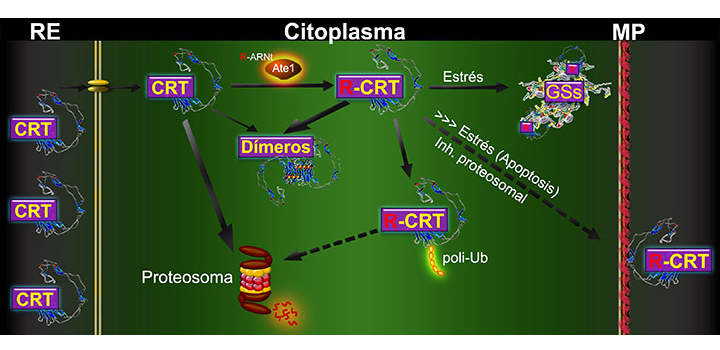
Post-translational arginylation has been suggested to target proteins for proteasomal degradation. The degradation mechanism for arginylated calreticulin (R-CRT) localized in the cytoplasm is unknown. To evaluate the effect of arginylation on CRT stability, we examined the metabolic fates and degradation mechanisms of cytoplasmic CRT and R-CRT in NIH 3T3 and CHO cells. Both CRT isoforms were found to be proteasomal substrates, but the half-life of R-CRT (2 h) was longer than that of cytoplasmic CRT (0.7 h). Arginylation was not required for proteasomal degradation of CRT, although R-CRT displays ubiquitin modification. A CRT mutant incapable of dimerization showed reduced metabolic stability of R-CRT, indicating that R-CRT dimerization may protect it from proteasomal degradation. Our findings, taken together, demonstrate a novel function of arginylation: increasing the half-life of CRT in cytoplasm.
Authors: Goitea VE, Hallak ME.
Article: Goitea VE et al., J. Biol. Chem, 2015, 290:16403-16414.