Mariani ME et al. 2015, Chem. Phys. Lipids
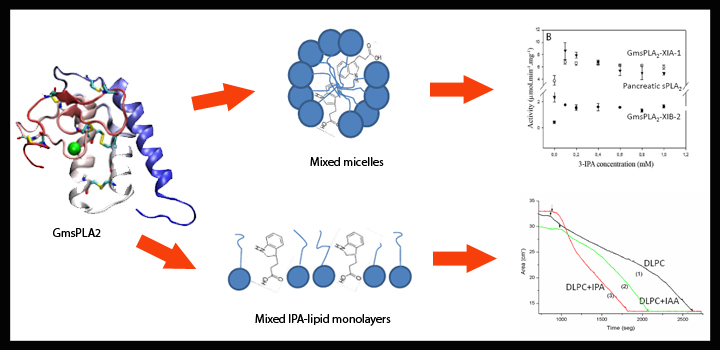
Secretory phospholipase A2 (sPLA2) are soluble enzymes that catalyze the conversion of phospholipids to lysophospholipids and free fatty acids at membrane interfaces. The effect of IAA and IPA auxins over the activity of recombinant sPLA2 isoforms from Glycine max was studied using membrane model systems including mixed micelles and Langmuir lipid monolayers. Both phytohormones stimulate the activity of both plant sPLA2 using DLPC/Triton mixed micelles as substrate. To elucidate the mechanism of action of the phytohormones, we showed that both auxins are able to self-penetrate lipid monolayers and cause an increment in surface pressure and an expansion of lipid/phytohormone mixed interfaces. The stimulating effect of auxins over phospholipase A2 activity was still present when using Langmuir mixed monolayers as organized substrate regardless of sPLA2 source (plant or animal). All the data suggest that the stimulating effect of auxins over sPLA2 is due to a more favorable interfacial environment rather to a direct effect over the enzyme.
Authors: Marian ME, Madoery RR, Fidelio GD.
Article: Mariani ME et al., Chem. Phys. Lipids, 2015, 189:1-6